 Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.  Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.
 Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free!  Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free!
 Thank you for choosing ACROBiosystems. Would you rate our product and service?
Thank you for choosing ACROBiosystems. Would you rate our product and service?  Thank you for choosing ACROBiosystems. Would you rate our product and service?
Thank you for choosing ACROBiosystems. Would you rate our product and service?
 Here come GMP Grade Cytokines!Free Sample is available!
Here come GMP Grade Cytokines!Free Sample is available!  Here come GMP Grade Cytokines!Free Sample is available!
Here come GMP Grade Cytokines!Free Sample is available!
Designed under ISO 9001:2015 and ISO 13485:2016
Manufactured and QC tested under a GMP compliance factory
Animal-Free materials
Beta-lactam materials free
Batch-to-batch consistency
Stringent quality control tests

This protein carries no "tag".
The protein has a calculated MW of 35.5 kDa. The protein migrates as 75 kDa±5 kDa when calibrated against Star Ribbon Pre-stained Protein Marker under reducing (R) condition (SDS-PAGE) due to glycosylation.
>95% as determined by SDS-PAGE.
Lyophilized from 0.22 μm filtered solution in 20 mM NaAc-HAc, pH5.0 with protectants.
Contact us for customized product form or formulation.
This product is supplied and shipped with blue ice, please inquire the shipping cost.
Upon receipt, store it immediately at -20°C or lower for long term storage.
Please avoid repeated freeze-thaw cycles.
This product is stable after storage at:
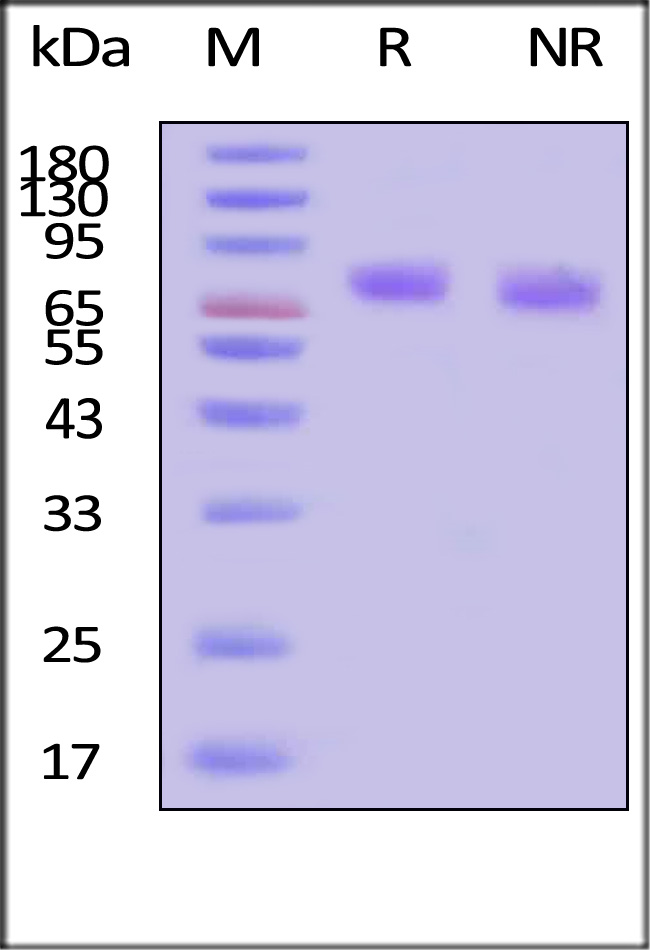
GMP Human Thrombopoietin Protein on SDS-PAGE under reducing (R) and non-reducing (NR) conditions. The gel was stained with Coomassie Blue. The purity of the protein is greater than 95% (With Star Ribbon Pre-stained Protein Marker).
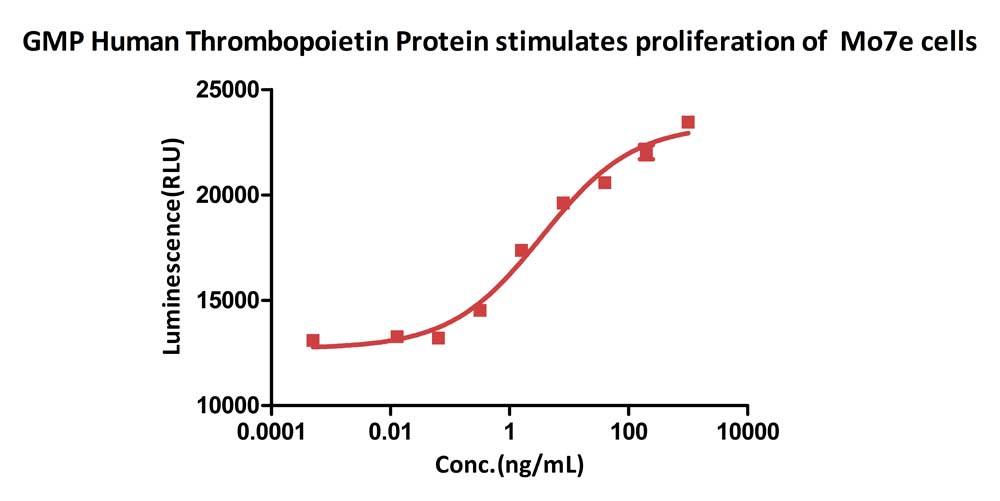
GMP Human Thrombopoietin Protein (Cat. No. GMP-THNH25) stimulates proliferation of Mo7e cells. The specific activity of GMP Human Thrombopoietin Protein is >1.00ⅹ10^7 IU/mg, which is calibrated against human TPO Standard (NIBSC code: 03/124) (QC tested).

The activity of GMP Human Thrombopoietin Protein (Cat. No. GMP-THNH25) was higher than other competing products.

GMP Human SCF Protein (Cat. No. GMP-SCFH25), GMP Human Thrombopoietin Protein (Cat. No. GMP-THNH25), GMP Human Flt-3 Ligand Protein (Cat. No. GMP-FLLH28), GMP Human FGF basic Protein (Cat. No. GMP-FGCH17) and GMP Human VEGF165 Protein (Cat. No. GMP-VE5H23) could significantly promote the iPSC differentiation to HSPCSs after 14 days, highly expressed hematopoietic stem cell markers CD34 and CD45.

GMP Human Thrombopoietin Protein (Cat. No. GMP-THNH25) is crucial for the expansion of human CD34+ hematopoietic cells cultured with medium containing GMP Human SCF Protein (Cat. No. GMP-SCFH25) and GMP Human Flt-3 Ligand Protein (Cat. No. GMP-FLLH28). Cell viability was checked using a luminescent cell viability detection reagent.

The Cell based assay shows that GMP Human Thrombopoietin Protein (Cat. No. GMP-THNH25) is stable at 37°C for 24 hours.

The Cell based assay shows that GMP Human Thrombopoietin Protein (Cat. No. GMP-THNH25) is stable at 4°C for 90 days.

The Cell based assay shows that GMP Human Thrombopoietin Protein (Cat. No. GMP-THNH25) is stable after freezing and thawing 3 times.

The Cell based assay shows batch-to-batch consistency between Acro's GMP and PG Thrombopoietin.
ACROBiosystems GMP grade products are produced under a quality management system and in compliance with relevant guidelines: Ph. Eur General Chapter 5.2.12 Raw materials of biological origin for the production of cell-based and gene therapy medicinal products; USP<92>Growth Factors and Cytokines Used in Cell Therapy Manufacturing; USP<1043>Ancillary Materials for Cell, Gene, and Tissue-Engineered Products; ISO/TS 20399-1:2018, Biotechnology - Ancillary Materials Present During the Production of Cellular Therapeutic Products.
ACROBiosystems Quality Management System Contents:
Designed under ISO 9001:2015 and ISO 13485:2016, Manufactured and QC tested under a GMP compliance factory.
Animal-Free materials
Materials purchased from the approved suppliers by QA
ISO 5 clean rooms and automatic filling equipment
Qualified personnel
Quality-related documents review and approve by QA
Fully batch production and control records
Equipment maintenance and calibration
Validation of analytical procedures
Stability studies conducted
Comprehensive regulatory support files
Request For Regulatory Support Files(RSF)
ACROBiosystems provide rigorous quality control tests (fully validated equipment, processes and test methods) on our GMP grade products to ensure that they meet stringent standards in terms of purity, safety, activity and inter-batch stability, and each bulk QC lot mainly contains the following specific information:
SDS-PAGE
Protein content
Endotoxin level
Residual Host Cell DNA content
Residual Host Cell Protein content
Biological activity analysis
Microbial testing
Mycoplasma testing
In vitro virus assay
Batch-to-batch consistency
価格(JPY) : 232,400
価格(JPY) : 1,282,400
価格(JPY) : 1,750,000

ACROBiosystemsは、二重特異性抗体の治療薬への臨床開発を加速するために、高い生物活性を持つ均一なCD3δ/CD3εおよびCD3γ/CD3εタンパク質のシリーズを開発しました。

We offer a wide range of cell and gene therapy solutions starting from discovery to the clinic. Explore our wide range of proteins, antibodies, kits, and other assays to accelerate the development of your cell and gene therapy.

Organoid Toolbox は、即使用可能のオルガノイド、オルガノイド分化ツールキット、創薬プロジェクトの進行を加速する、さまざまなサービスを含むオルガノイドソリューションのことです。

IL-15、IL-7、IL-21など、高品質のGMP準拠サイトカイン製品を提供しており、免疫細胞治療薬の臨床研究をサポートし、医薬品規制当局の承認を加速できます。

注目されている50以上のCAR-Tターゲットがあり、CARの発現の検出のために設計された抗原タンパク質をフローサイトメトリーで検証し、CARの発現が高い特異性で検出できます。ロット間の一貫性が保証されています。また、PE / FITC標識タンパク質を利用することで、ワンステップ染色でCARの発現を高い特異性でバックグラウンドなしに検出できます。

CD20、Claudin18.2、CD133、GPRC5D、CCR5、CCR8などの安定で高活性な全長型膜貫通タンパク質は、免疫学、ELISA、SPR、BLI、細胞実験、CAR陽性率検出に利用できます。VLP、界面活性剤、ナノディスクなどのテクニカルプラットフォームによって複数回膜貫通タンパク質の医薬品創薬を促進できます。

GMP準拠サイトカイン、高品質の細胞活性化/増幅試薬、遺伝子改変試薬/酵素、CAR検出試薬などの製品を提供し、細胞、遺伝子治療用に全体的なソリューションを提供し、創薬から臨床研究までのすべての段階をサポートします。

これまでに知られている免疫チェックポイント分子をほぼ製品化しており、様々なタグの製品群を提供できます。天然高分子の構造はMALSによって、生物活性はELISA/SPR/BLI/FACSなどによって検証済みです。またBiotin/FITCなどの標識も選択でき、抗体のハイスループットスクリーニングに利用できます。

弊社ACROBiosystemsはADC医薬品の開発のサポートに取り組み、注目されている様々なターゲットのために、異なる種類とタグの製品が開発され、高い純度と親和性が特長です。免疫、抗体スクリーン、SPR、細胞活性検査などの実験に利用できます。Protocolも無償で提供いたします。

すべての分子のFc受容体タンパク質だけでなく、一般的な変異体やビオチン標識タイプも含まれております。お客様のモノクローナル抗体の開発をサポートします。

インターロイキン、成長因子、ケモカイン、TNF など、様々なサイトカインターゲットの自然なコンフォメーションを確保するために HEK293 によって発現されます。SDS-PAGE/HPLC/SEC-MALS によって高純度は確認され、高い生物活性は ELISA/SPR/BLI によって確認されています。

AneuroはACROBiosystemsが神経科学研究のために設計した製品群のブランドです。神経科学研究を推進するための治療・診断用研究タンパク質、PFF、組み換え神経因子など、高品質の重要なタンパク質を提供しております。
This web search service is supported by Google Inc.