 Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.  Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.
 Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free!  Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free!
 Thank you for choosing ACROBiosystems. Would you rate our product and service?
Thank you for choosing ACROBiosystems. Would you rate our product and service?  Thank you for choosing ACROBiosystems. Would you rate our product and service?
Thank you for choosing ACROBiosystems. Would you rate our product and service?
 Here come GMP Grade Cytokines!Free Sample is available!
Here come GMP Grade Cytokines!Free Sample is available!  Here come GMP Grade Cytokines!Free Sample is available!
Here come GMP Grade Cytokines!Free Sample is available!
| Project Name | Modality | Therapeutic Area | Indications | Stage | Right Available |
| IL5 mAb | Monospecific antibody | Immunological disease | Asthma | Phase II | Global (except China) |
| 製造番号 | 種類 | 製品説明 | 構造 | 純度 | 特徴 |
|---|---|---|---|---|---|
| IL5-H5214 | Human | Human IL-5 Protein, premium grade | 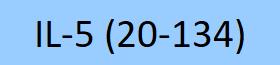 |
|
|
| EP-128 | Human | IL-5[Biotinylated]:IL-5Rα Inhibitor Screening ELISA Kit | |||
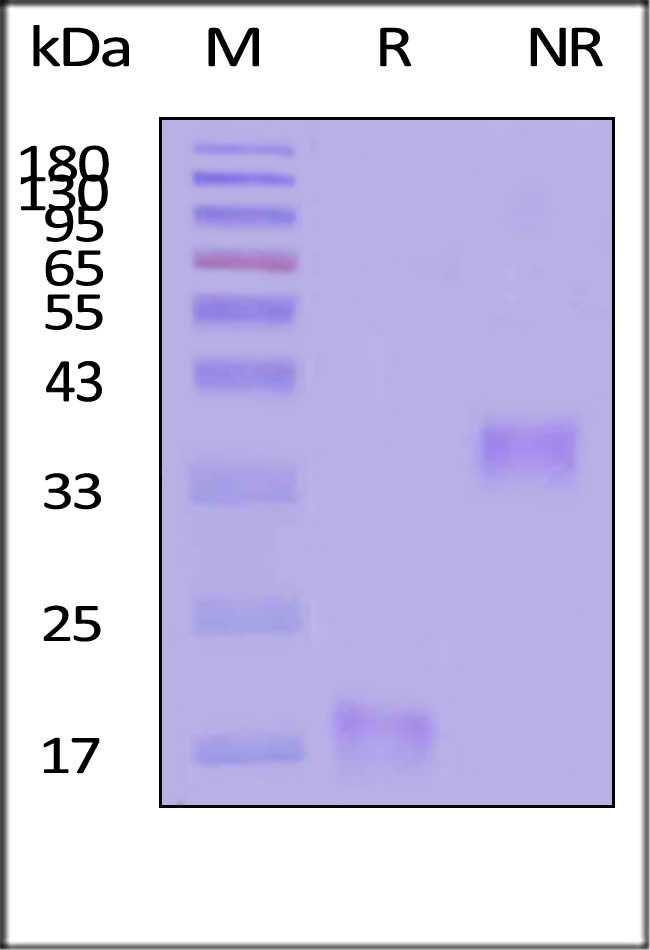
| IL5-C52H3 | Cynomolgus | Cynomolgus IL-5 Protein, His Tag |  |
|
|
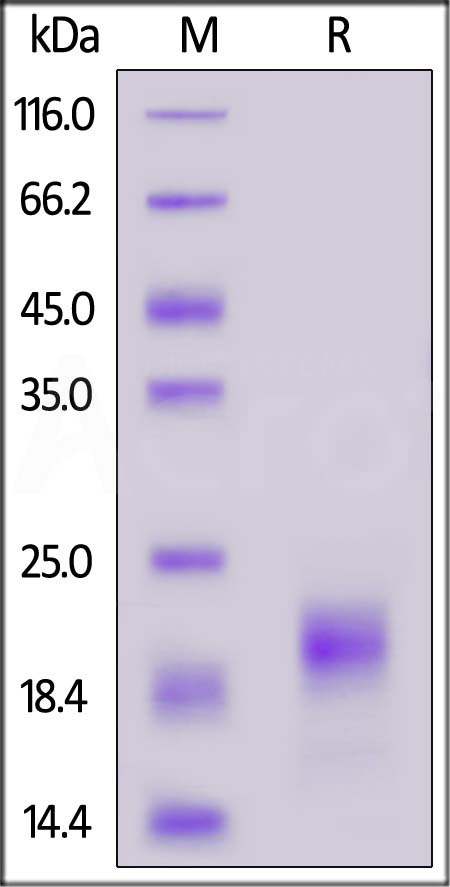
| IL5-M52H3 | Mouse | Mouse IL-5 Protein, His Tag (MALS verified) |  |

|
|
| IL5-R52H4 | Rabbit | Rabbit IL-5 Protein, His Tag |  |
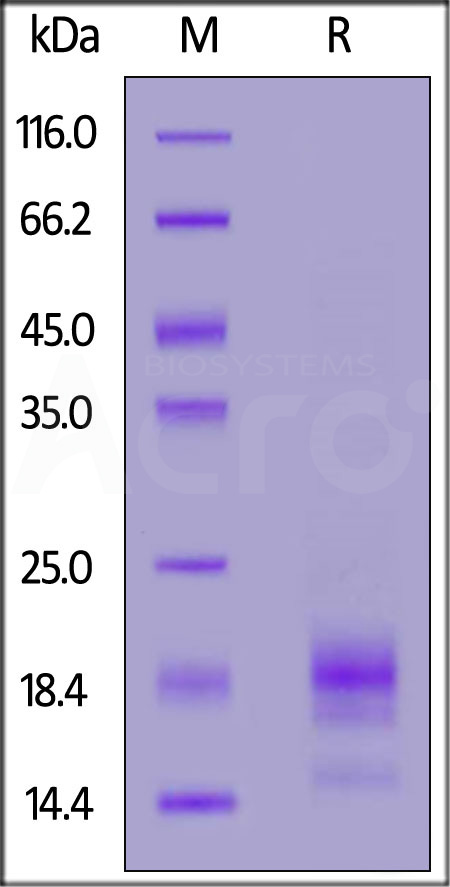
|
|
| IL5-H82Q5 | Human | Biotinylated Human IL-5 Protein, His,Avitag™ |  |

|
|
| IL5-H52H3 | Human | Human IL-5 Protein, His Tag |  |

|
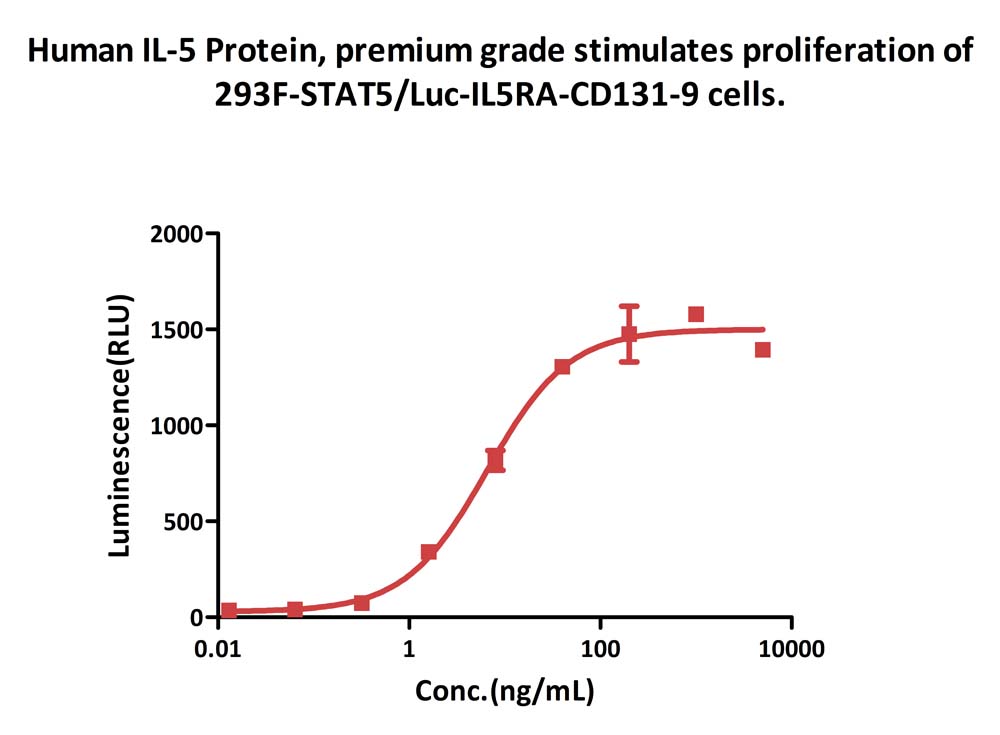
Human IL-5 Protein, premium grade (Cat. No. IL5-H5214) stimulates proliferation of 293F-STAT5/Luc-IL5RA-CD131-9 cells. The specific activity of Human IL-5 Protein, premium grade is > 3.00ⅹ10^6 IU/mg, which is calibrated against human IL-5 WHO International Standard (NIBSC code: 90/586) (QC tested).
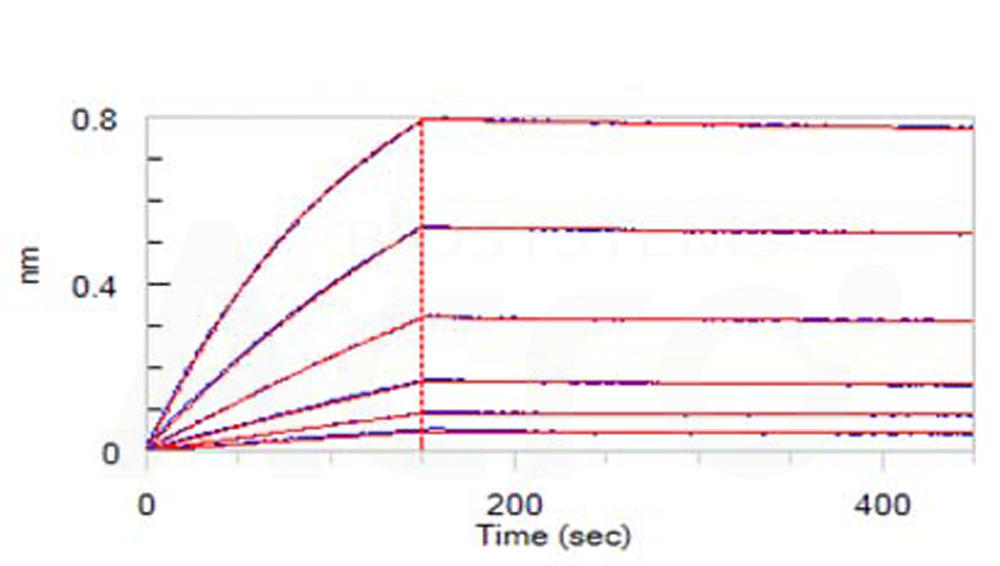
Loaded Human IL-5 Protein, His Tag (Cat. No. IL5-H52H3) on NTA Biosensor, can bind Human IL-5 R alpha, Fc Tag with an affinity constant of 4.63 nM as determined in BLI assay (ForteBio Octet Red96e) (Routinely tested).
![IL-5[Biotinylated]:IL-5Rα Inhibitor Screening ELISA KitIL-5[Biotinylated]:IL-5Rα Inhibitor Screening ELISA Kit (Cat. No. EP-128) ELISA bioactivity](/static/main/products/images/elisa/EP-128-E1.jpg)
INHIBITION OF IL-5 [BIOTINYLATED]: IL-5 R ALPHA BINDING BY Human IL-5
Serial dilutions of Human IL-5(Catalog # EP128-C03) (1:1 serial dilution, from 10 μg/mL to 0.020 μg/mL (666.67-0.65 nM)) was added into IL-5 R alpha: IL-5-Biotin binding reactions. The assay was performed according to the above-described protocol. Background was subtracted from data points prior to log transformation and curve fitting (QC tested).
| Name | Research Code | Research Phase | Company | First Brand Name | First Approved Country | First Indication | First Approved Company | First Approved Date | Indications | Clinical Trials |
|---|---|---|---|---|---|---|---|---|---|---|
| Reslizumab | TRFK-5; CDP-835; SCH-5570; CEP-38072; CTx-55700; SCH-55700 | Approved | Teva Pharmaceutical Industries Ltd | Cinqair, Cinquil, Cinqaero | EU | Asthma | Teva Bv | 2016-03-23 | Loiasis; Hypereosinophilic Syndrome; Pulmonary Eosinophilia; Eosinophilic Esophagitis; Asthma; Bronchitis | Details |
| Mepolizumab | SB-240563; 240563 | Approved | Glaxosmithkline Plc | Bosatria, Nucala, 美泊利珠单抗 | EU | Eosinophilic Granuloma; Nasal Polyps; Hypereosinophilic Syndrome; Sinusitis | Glaxosmithkline Trading Services Ltd | 2015-11-04 | Hypereosinophilic Syndrome; Granulomatosis with Polyangiitis; Churg-Strauss Syndrome; Virus Diseases; Nasal Polyps; Chronic Urticaria; Eosinophilia; Eosinophilic Esophagitis; Asthma; Eosinophilic Granuloma; Bronchitis; Sinusitis; Pulmonary Disease, Chronic Obstructive; Fasciitis; Dermatitis, Atopic; Angioedema | Details |
| Name | Research Code | Research Phase | Company | Indications | Clinical Trials |
|---|---|---|---|---|---|
| long-acting anti-IL-5 mAb (GSK) | Phase 1 Clinical | Glaxosmithkline Plc | Asthma | Details | |
| Mepolizumab biosimilar (CTTQ Pharma) | Phase 1 Clinical | Nanjing Shunxin Pharmaceuticals Co Ltd Of Chiatai Tianqing Pharmaceutical Group | Hypereosinophilic Syndrome; Vasculitis; Nasal Polyps; Asthma; Eosinophilic Granuloma; Sinusitis | Details | |
| Mepolizumab biosimilar(Bio Thera Solutions) | Phase 1 Clinical | Bio-Thera Solutions Ltd | Autoimmune Diseases; Nasal Polyps; Sinusitis | Details | |
| SHR-1703 | SHR-1703 | Phase 2 Clinical | Suzhou Suncadia Biopharmaceuticals Co Ltd, Jiangsu Hengrui Medicine Co Ltd, Shanghai Hengrui Pharmaceutical Co Ltd | Asthma | Details |
| Recombinant anti-IL-5 humanized monoclonal antibody (Shanghai CP Guojian) | 610 | Phase 2 Clinical | Shanghai Cp Guojian Pharmaceutical Co Ltd | Autoimmune Diseases; Asthma | Details |
| Depemokimab | GSK-294; GSK3511294; GSK294; GSK-3511294 | Phase 3 Clinical | Glaxosmithkline Plc | Hypereosinophilic Syndrome; Churg-Strauss Syndrome; Nasal Polyps; Vasculitis; Asthma; Eosinophilic Granuloma; Sinusitis | Details |
This web search service is supported by Google Inc.