 Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.  Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.
 Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free!  Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free!
 Thank you for choosing ACROBiosystems. Would you rate our product and service?
Thank you for choosing ACROBiosystems. Would you rate our product and service?  Thank you for choosing ACROBiosystems. Would you rate our product and service?
Thank you for choosing ACROBiosystems. Would you rate our product and service?
 Here come GMP Grade Cytokines!Free Sample is available!
Here come GMP Grade Cytokines!Free Sample is available!  Here come GMP Grade Cytokines!Free Sample is available!
Here come GMP Grade Cytokines!Free Sample is available!

Immobilized Native Human Collagen I protein at 10 μg/mL (100 μL/well) can bind Biotinylated Human LAIR-1, Fc,Avitag (Cat. No. LA1-H82F5) with a linear range of 0.01-0.625 μg/mL (QC tested).
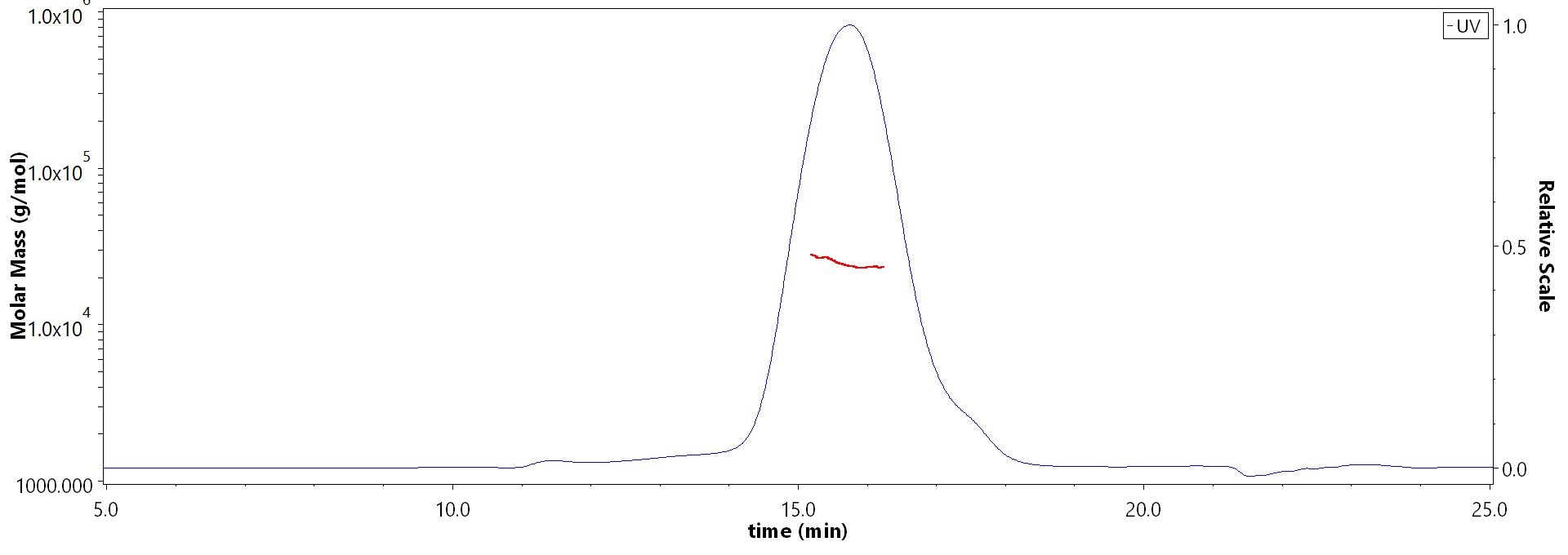
The purity of Mouse LAIR1 Protein, His Tag (Cat. No. LA1-M52H3) is more than 90% and the molecular weight of this protein is around 20-28 kDa verified by SEC-MALS.
| Name | Research Code | Research Phase | Company | Indications | Clinical Trials |
|---|---|---|---|---|---|
| NGM-438 | NGM-438 | Phase 1 Clinical | Merck & Co Inc, Ngm Biopharmaceuticals Inc | Ovarian Neoplasms; Solid tumours; Squamous Cell Carcinoma of Head and Neck; Esophageal Neoplasms; Carcinoma, Renal Cell; Stomach Neoplasms; Carcinoma, Transitional Cell; Pancreatic Neoplasms; Mesothelioma; Cholangiocarcinoma; Breast Neoplasms; Prostatic Neoplasms; Colorectal Neoplasms; Melanoma; Carcinoma, Non-Small-Cell Lung; Uterine Cervical Neoplasms | Details |
| NC-410 | NC-410 | Phase 2 Clinical | Nextcure Inc | Ovarian Neoplasms; Solid tumours; Stomach Neoplasms; Colorectal Neoplasms | Details |
| NGM-438 | NGM-438 | Phase 1 Clinical | Merck & Co Inc, Ngm Biopharmaceuticals Inc | Ovarian Neoplasms; Solid tumours; Squamous Cell Carcinoma of Head and Neck; Esophageal Neoplasms; Carcinoma, Renal Cell; Stomach Neoplasms; Carcinoma, Transitional Cell; Pancreatic Neoplasms; Mesothelioma; Cholangiocarcinoma; Breast Neoplasms; Prostatic Neoplasms; Colorectal Neoplasms; Melanoma; Carcinoma, Non-Small-Cell Lung; Uterine Cervical Neoplasms | Details |
| NC-410 | NC-410 | Phase 2 Clinical | Nextcure Inc | Ovarian Neoplasms; Solid tumours; Stomach Neoplasms; Colorectal Neoplasms | Details |
This web search service is supported by Google Inc.