 Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.  Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.
 Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free!  Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free!
 Thank you for choosing ACROBiosystems. Would you rate our product and service?
Thank you for choosing ACROBiosystems. Would you rate our product and service?  Thank you for choosing ACROBiosystems. Would you rate our product and service?
Thank you for choosing ACROBiosystems. Would you rate our product and service?
 Here come GMP Grade Cytokines!Free Sample is available!
Here come GMP Grade Cytokines!Free Sample is available!  Here come GMP Grade Cytokines!Free Sample is available!
Here come GMP Grade Cytokines!Free Sample is available!
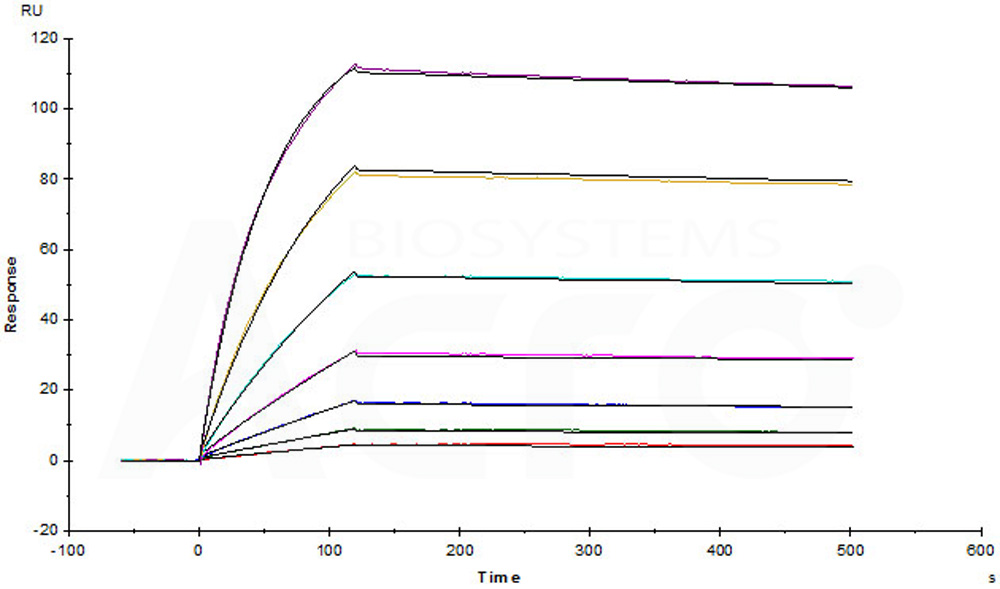
Captured Human RANK, Mouse IgG2a Fc Tag, low endotoxin (Cat. No. RAK-H5251) on CM5 chip via Anti-Mouse antibodies surface can bind Human TNFSF11, Fc Tag (Cat. No. RAL-H5265) with an affinity constant of 0.574 nM as determined in a SPR assay (Biacore T200) (Routinely tested).
| Name | Research Code | Research Phase | Company | First Brand Name | First Approved Country | First Indication | First Approved Company | First Approved Date | Indications | Clinical Trials |
|---|---|---|---|---|---|---|---|---|---|---|
| Denosumab biosimilar(Reliance Life Sciences) | R-TPR-045 | Approved | Reliance Life Sciences | DenosuRel | India | Osteoporosis, Postmenopausal; Bone metastases | Reliance Life Sciences | 2022-02-01 | Bone metastases; Osteoporosis, Postmenopausal | Details |
| Denosumab biosimilar (Boan Biopharma/Luye) | LY-01011; BA-1102; BA-6101; LY-06006 | Approved | Shandong Boan Biotechnology Co Ltd, Luye Pharma Group Ltd | Mainland China | Osteoporosis, Postmenopausal | Shandong Boan Biotechnology Co Ltd | 2022-11-10 | Bone metastases; Solid tumours; Osteoporosis; Osteoporosis, Postmenopausal; Neoplasms; Bone Neoplasms; Neoplasm Metastasis | Details | |
| Denosumab | NSC-744010; AMG-162; ENZ-215 | Approved | Amgen Inc | Prolia, Xgeva, Pralia, Ranmark | Mainland China | Osteoporosis, Postmenopausal | Amgen Inc | 2010-05-26 | Lung Neoplasms; Prostatic Neoplasms, Castration-Resistant; Feeding and Eating Disorders; Bone Cysts, Aneurysmal; Giant Cell Tumors; Prostatic Neoplasms; Breast Neoplasms; Arthropathy, Neurogenic; Osteosarcoma; Osteogenesis Imperfecta; Giant Cell Tumor of Bone; Breast Diseases; Bone Neoplasms; Multiple Myeloma; Fractures, Bone; Lymphoma; Granuloma, Giant Cell; Carcinoma, Squamous Cell; Lymphoma, Non-Hodgkin; Thyroid Neoplasms; Osteoblastoma; Carcinoma, Non-Small-Cell Lung; Anorexia Nervosa; Crohn Disease; Neoplasm Metastasis; Fibroma; Pain; Hematologic Neoplasms; Endocrine Gland Neoplasms; Kidney Neoplasms; Cataract; Solid tumours; Hematopoietic stem cell transplantation (HSCT); Head and Neck Neoplasms; Osteoporosis; Parathyroid Neoplasms; Carcinoma; Bone Diseases, Metabolic; Fibrous Dysplasia of Bone; Bone metastases; Osteoporosis, Postmenopausal; Chondroblastoma; Colonic Neoplasms; Neoplasms; Hypercalcemia; Kidney Diseases; Arthritis, Rheumatoid; Smoldering Multiple Myeloma; Hodgkin Disease; Carcinoma, Ovari | Details |
| Name | Research Code | Research Phase | Company | Indications | Clinical Trials |
|---|---|---|---|---|---|
| Recombinant human anti-RANKL momoclonal antibody (Qilu Pharmaceutical) | Phase 3 Clinical | Qilu Pharmaceutical Co Ltd | Osteoporosis, Postmenopausal; Neoplasms | Details | |
| Recombinant human anti-RANKL antibody (Innovent Biologics) | IBI-307 | Phase 1 Clinical | Innovent Biologics(Suzhou) Co Ltd | Osteoporosis | Details |
| Denosumab biosimilar (Intas Biopharmaceuticals) | Phase 3 Clinical | Intas Biopharmaceuticals | Osteoporosis, Postmenopausal | Details | |
| Denosumab biosimilar (Alphamab) | KN-012 | Phase 3 Clinical | Osteoporosis, Postmenopausal | Details | |
| LZM-004 | LZM-004 | Phase 1 Clinical | Livzon(Group) Pharmaceutical Factory | Bone metastases | Details |
| Recombinant human anti-RANKL antibody (Hisun Pharma) | HS629 | Phase 1 Clinical | Zhejiang Hisun Pharmaceutical Co Ltd | Solid tumours; Bone metastases | Details |
| Denosumab biosimilar(Fresenius Kabi) | FKS-518 | Fresenius Kabi Swissbiosim Gmbh | Details | ||
| GB-223 | GB-223 | Phase 1 Clinical | Genor Biopharma Co Ltd | Solid tumours; Bone metastases; Osteoporosis, Postmenopausal; Giant Cell Tumor of Bone | Details |
| CEP-37251 | ART-010; EGX-010; CEP-37251 | Teva | Details | ||
| Denosumab biosimilar (AryoGen) | Phase 3 Clinical | Aryogen Biopharma | Osteoporosis | Details | |
| KP-46 | KP-46; FFC-11; LX-001; AP-002 | Phase 2 Clinical | University Of Heidelberg, Meram | Solid tumours | Details |
| Denosumab biosimilar(Mabxience) | Phase 3 Clinical | Mabxience Sa | Osteoporosis, Postmenopausal | Details | |
| Denosumab biosimilar (Samsung Bioepis) | SB-16 | Phase 3 Clinical | Samsung Bioepis Co Ltd | Osteoporosis, Postmenopausal | Details |
| MV-088 | MV-088 | Phase 1 Clinical | Kpc Pharmaceuticals Inc | Osteoporosis, Postmenopausal | Details |
| Denosumab biosimilar(Alvotech Swiss) | AVT-03 | Phase 3 Clinical | Alvotech Swiss Ag | Osteoporosis, Postmenopausal | Details |
| Denosumab biosimilar (Hualan Biological Engineering) | Phase 1 Clinical | Hualan Genetic Engineering Co Ltd | Solid tumours; Osteoporosis, Postmenopausal | Details | |
| Denosumab biosimilar(Shanghai Hansoh) | HS-20090; HS-20090-2 | Phase 3 Clinical | Shanghai Hansoh Biomedical Co Ltd, Jiangsu Hansoh Pharmaceutical Group Co Ltd | Solid tumours; Bone metastases; Neoplasms; Osteoporosis, Postmenopausal; Bone Diseases; Hypercalcemia; Multiple Myeloma; Giant Cell Tumor of Bone; Nutritional and Metabolic Diseases | Details |
| RGB-14-P | RGB-14-P | Phase 3 Clinical | Gedeon Richter Plc | Osteoporosis, Postmenopausal | Details |
| Denosumab biosimiliar (Celltrion) | CT-P41 | Phase 3 Clinical | Celltrion Inc | Osteoporosis, Postmenopausal | Details |
| Denosumab biosimilar (Sandoz) | GP-2411 | Phase 3 Clinical | Novartis Pharma Ag, Sandoz | Osteoporosis, Postmenopausal | Details |
| Denosumab biosimilar (Henlius ) | HLX-14; HLX14 | Phase 3 Clinical | Shanghai Henlius Biotech Co Ltd | Osteoporosis, Postmenopausal | Details |
This web search service is supported by Google Inc.