 Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.  Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.
 Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free!  Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free!
 Thank you for choosing ACROBiosystems. Would you rate our product and service?
Thank you for choosing ACROBiosystems. Would you rate our product and service?  Thank you for choosing ACROBiosystems. Would you rate our product and service?
Thank you for choosing ACROBiosystems. Would you rate our product and service?
 Here come GMP Grade Cytokines!Free Sample is available!
Here come GMP Grade Cytokines!Free Sample is available!  Here come GMP Grade Cytokines!Free Sample is available!
Here come GMP Grade Cytokines!Free Sample is available!
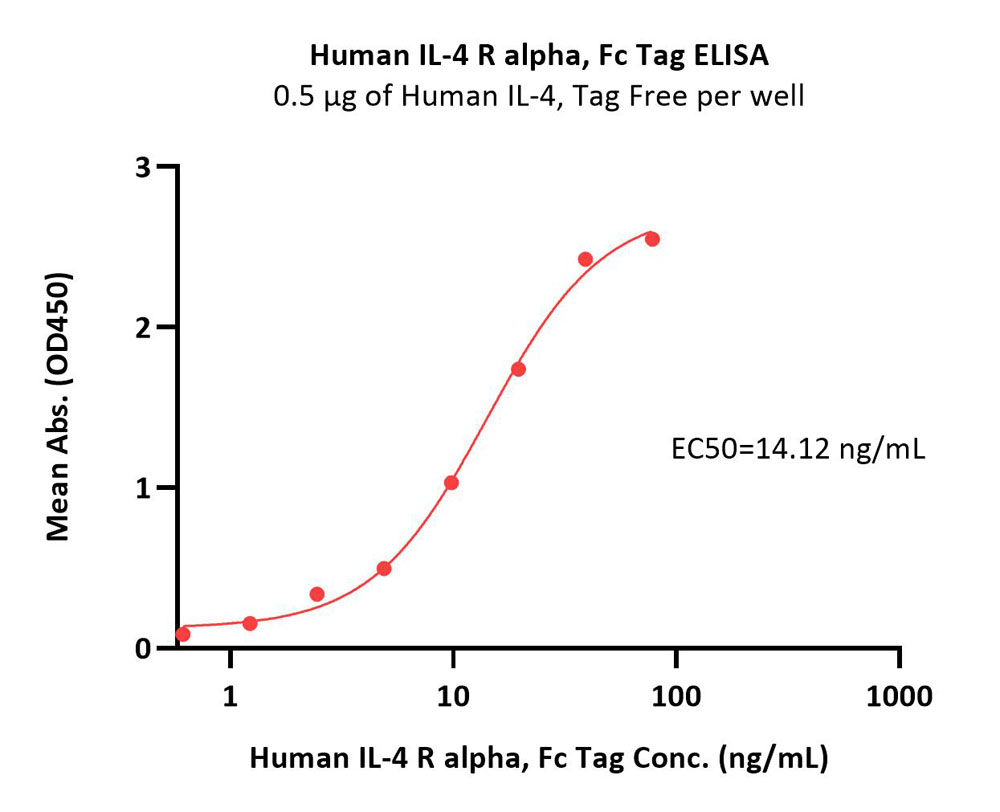
Immobilized Human IL-4, premium grade (Cat. No. IL4-H4218) at 5 μg/mL (100 μL/well)can bind Human IL-4 R alpha, Fc Tag (Cat. No. ILR-H5253) with a linear range of 1-20 ng/mL (QC tested).

The purity of Biotinylated Human IL-4 R alpha, Fc,Avitag (Cat. No. ILR-H82F4) is more than 90% and the molecular weight of this protein is around 120-147 kDa verified by SEC-MALS.
| Name | Research Code | Research Phase | Company | First Brand Name | First Approved Country | First Indication | First Approved Company | First Approved Date | Indications | Clinical Trials |
|---|---|---|---|---|---|---|---|---|---|---|
| Dupilumab | SAR-231893; REGN-668 | Approved | Sanofi, Regeneron Pharmaceuticals Inc | 达必妥, Dupixent | United States | Prurigo | Regeneron Pharmaceuticals Inc | 2017-03-28 | Hypersensitivity, Immediate; Prostatic Neoplasms; Prurigo; Neurodermatitis; Asthma; Pemphigoid, Bullous; Urticaria; Sinusitis; Pulmonary Disease, Chronic Obstructive; Conjunctivitis, Allergic; Peanut Hypersensitivity; Scleroderma, Localized; Keratoconjunctivitis; Keloid; Dermatitis, Atopic; Eczema; Angioedema; Hypersensitivity; Respiratory Tract Diseases; Eosinophilic gastroenteritis (EG); Meningitis; Pruritus; Alopecia Areata; Chronic Urticaria; Nasal Polyps; Respiration Disorders; Milk Hypersensitivity; Aspergillosis, Allergic Bronchopulmonary; Coronavirus Disease 2019 (COVID-19); Dermatitis; Skin Diseases, Eczematous; Skin Diseases; Genetic Diseases, Inborn; Sleep Apnea Syndromes; Eosinophilic Esophagitis; Rhinitis, Allergic | Details |
| Name | Research Code | Research Phase | Company | Indications | Clinical Trials |
|---|---|---|---|---|---|
| Sorafenib Tosylate/MG-K10 | MG-D-1609 | Phase 2 Clinical | Solid tumours | Details | |
| AMG-317 | AMG-317 | Amgen Inc | Details | ||
| QX-005N | QX-005N | Phase 2 Clinical | Qyuns Therapeutics Co Ltd | Chronic Urticaria; Asthma; Prurigo; Sinusitis; Dermatitis, Atopic | Details |
| Elarekibep | PRS-060/AZD1402; PRS-060; AZD-1402 | Phase 2 Clinical | University Of Melbourne, Pieris Pharmaceuticals | Asthma | Details |
| SHR-1819 | SHR-1819 | Phase 2 Clinical | Jiangsu Hengrui Medicine Co Ltd | Asthma; Dermatitis, Atopic | Details |
| Manfidokimab | AK-120 (Akeso ) | Phase 2 Clinical | Zhongshan Akeso Biopharma Co Ltd | Asthma; Dermatitis, Atopic | Details |
| HY-1770 | HY-1770; HY1770; HT-17; HT17 | Phase 1 Clinical | Suzhou Pharmavan Co Ltd | Dermatitis, Atopic; Plaque psoriasis | Details |
| Recombinant anti-IL-4Rα humanized monoclonal antibody (Sansheng Guojian) | 611; 611 Q2W; 611 Q4W | Phase 2 Clinical | Shanghai Cp Guojian Pharmaceutical Co Ltd | Dermatitis, Atopic | Details |
| LQ-036 | LQ-036 | Phase 1 Clinical | Shanghai Novamab Biopharmaceuticals Co Ltd | Asthma | Details |
| BA-2101 | BA-2101; BA2101 | Phase 1 Clinical | Shandong Boan Biotechnology Co Ltd | Nasal Polyps; Chronic Urticaria; Sinusitis; Prurigo; Asthma; Dermatitis, Atopic | Details |
| MG-K10 | MG-K10; MG-010; MG-K-10; BC-005 | Phase 2 Clinical | Dragonboat Biopharmaceutical, Shanghai Mabgeek Biotechnology Co Ltd | Asthma; Dermatitis, Atopic | Details |
| CBP-201 | CBP-201 | Phase 3 Clinical | Suzhou Connect Biopharmaceuticals Ltd | Nasal Polyps; Nose Diseases; Asthma; Sinusitis; Status Asthmaticus; Dermatitis, Atopic | Details |
| CM-310 | CM-310 | Phase 3 Clinical | Keymed Biosciences Co Ltd, CSPC Pharmaceutical Group Ltd | Solid tumours; Pruritus; Nasal Polyps; Nose Diseases; Rhinitis, Allergic; Sinusitis; Asthma; Dermatitis, Atopic | Details |
This web search service is supported by Google Inc.