 Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.  Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.
 Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free!  Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free!
 Thank you for choosing ACROBiosystems. Would you rate our product and service?
Thank you for choosing ACROBiosystems. Would you rate our product and service?  Thank you for choosing ACROBiosystems. Would you rate our product and service?
Thank you for choosing ACROBiosystems. Would you rate our product and service?
 Here come GMP Grade Cytokines!Free Sample is available!
Here come GMP Grade Cytokines!Free Sample is available!  Here come GMP Grade Cytokines!Free Sample is available!
Here come GMP Grade Cytokines!Free Sample is available!

GMP Human IFN-gamma Protein (Cat. No. GMP-IFGH24) inhibits the proliferation of HT-29 cells. The specific activity of GMP Human IFN-gamma Protein is > 2.00×10^7 IU/mg, which is calibrated against human interferon gamma Standard (NIBSC code: 87/586) (QC tested).

Human IFN-gamma, premium grade (Cat. No. IFG-H4211) inhibits the proliferation of HT-29 cells. The specific activity of Human IFN-gamma, premium grade is > 2.00×10^7 IU/mg, which is calibrated against human interferon gamma Standard (NIBSC code: 87/586) (QC tested).
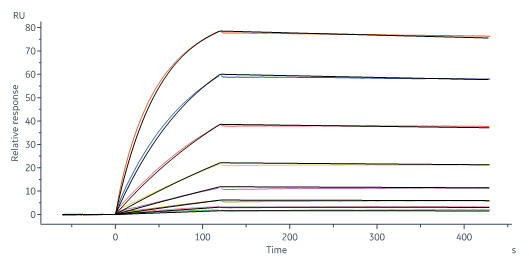
Biotinylated Monoclonal Anti-IFNγ Antibody, Mouse IgG1 (13E6H6) (Cat. No. IFN-BS138) captured on CM5 chip via anti-mouse antibodies surface can bind Human IFN-gamma, premium grade (Cat. No. IFG-H4211) with an affinity constant of 1.28 nM as determined in a SPR assay (Biacore 8K) (Routinely tested).

Monoclonal Anti-IFNγ antibody, Mouse IgG2a (8C5F8) (Cat. No. IFN-S120) captured on CM5 chip via anti-mouse antibodies surface can bind Human IFN-gamma, premium grade (Cat. No. IFG-H4211) with an affinity constant of 0.933 nM as determined in a SPR assay (Biacore 8K) (Routinely tested).
| Name | Research Code | Research Phase | Company | First Brand Name | First Approved Country | First Indication | First Approved Company | First Approved Date | Indications | Clinical Trials |
|---|---|---|---|---|---|---|---|---|---|---|
| Cadi-05 | Cadi-05 | Approved | National Institute Of Allergy And Infectious Diseases (Niaid) | Immuvac, Leprovac, Mycidac-C | India | Carcinoma, Non-Small-Cell Lung; Leprosy | null | 1998-01-01 | Leprosy; Carcinoma, Non-Small-Cell Lung | Details |
| Thalidomide | NSC-66847; NSC-527179; K-17; VP-02 | Approved | Celgene Corp | Talizer, Thalidomide Celgene, Thalidomide Pharmion, Synovir, Thalomid, Thaled | Japan | Leprosy, Lepromatous | Fujimoto Pharmaceutical | 1982-01-01 | Osteosarcoma; Leprosy, Lepromatous; Drug Resistant Epilepsy; Primary Myelofibrosis; Neuroectodermal Tumors, Primitive; Prostatitis; Colorectal Neoplasms; Lymphoma, Mantle-Cell; Sarcoma, Ewing; Retinoblastoma; Waldenstrom Macroglobulinemia; Cholangitis, Sclerosing; HIV Wasting Syndrome; Arachnoiditis; Adenocarcinoma, Clear Cell; Prostatic Neoplasms; Pancreatitis, Chronic; Lymphoma, Follicular; Sarcoma; Xerostomia; Burning Mouth Syndrome; Neoplasm Metastasis; Mycobacterium avium-intracellulare Infection; Vascular Malformations; Amyotrophic Lateral Sclerosis; Melanoma; Carcinoma, Hepatocellular; Leukemia, Lymphocytic, Chronic, B-Cell; Myelodysplastic-Myeloproliferative Diseases; Stomatitis; Erythema Nodosum; Anemia, Sideroblastic; Uterine Neoplasms; Lymphoma, Non-Hodgkin; Glioma; Angiodysplasia; Pelvic Pain; Appendiceal Neoplasms; Lung Neoplasms; Endometrial Neoplasms; Mycobacterium Infections; Gastric Antral Vascular Ectasia; Carcinoid Tumor; Lupus Erythematosus, Discoid; Stomatitis, Aphthous; Rhabdomyosarcoma; | Details |
| Emapalumab | NI-0501 | Approved | Novimmune Sa | Gamifant | Mainland China | Lymphohistiocytosis, Hemophagocytic | Sobi Pharmaceutical (Guangzhou) Co Ltd | 2018-11-20 | Macrophage Activation Syndrome; Still's Disease, Adult-Onset; Rare Diseases; Arthritis, Juvenile; Immune System Diseases; Lupus Erythematosus, Systemic; Lymphohistiocytosis, Hemophagocytic | Details |
| Name | Research Code | Research Phase | Company | Indications | Clinical Trials |
|---|---|---|---|---|---|
| OCH-NCNP1 | OCH-NCNP1 | Phase 2 Clinical | Keio University | Multiple Sclerosis; Crohn Disease | Details |
| AMG-811 | AMG-811 | Amgen Inc | Details | ||
| Tadekinig alfa (AB2 Bio) | Phase 3 Clinical | Ab2 Bio Ltd | Macrophage Activation Syndrome; Cytokine Release Syndrome; Lymphohistiocytosis, Hemophagocytic | Details | |
| Recombinant human interferon gamma adenovirus injection (Guangzhou Dabo Biological Products) | Phase 1 Clinical | Guangzhou Dabo Biological Products Co Ltd | Liver Neoplasms; Nasopharyngeal Neoplasms; Prostatic Neoplasms, Castration-Resistant | Details | |
| YH-32367 | ABL105; YH-32367 | Phase 2 Clinical | Abl Bio Inc | Solid tumours; Stomach Neoplasms; Breast Neoplasms | Details |
| SP-002 | SP-002; ASN-002; TG-1042 | Phase 2 Clinical | Solid tumours; Lymphoma, B-Cell; Carcinoma, Basal Cell; Skin Neoplasms; Basal Cell Nevus Syndrome; Melanoma | Details | |
| EI-001 | EI-001 | Phase 1 Clinical | Elixiron Immunotherapeutics Inc | Vitiligo | Details |
| KIO-100 | PP-001; KIO-101; KIO-100 | Phase 2 Clinical | 4sc Ag | Dry Eye Syndromes; Uveitis; Xerophthalmia; Keratoconjunctivitis | Details |
| PDS-0101 | PDS-0101B; PDS-0101C; PDS-0101; PDS-0101A; PDS-101 | Phase 2 Clinical | Pds Biotechnology Corporation, Merck Serono | Head and Neck Neoplasms; Anus Neoplasms; Papillomavirus Infections; Vulvar Neoplasms; Oropharyngeal Neoplasms; Carcinoma, Squamous Cell; Uterine Cervical Neoplasms | Details |
This web search service is supported by Google Inc.