 Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.  Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.
 Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free!  Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free!
 Thank you for choosing ACROBiosystems. Would you rate our product and service?
Thank you for choosing ACROBiosystems. Would you rate our product and service?  Thank you for choosing ACROBiosystems. Would you rate our product and service?
Thank you for choosing ACROBiosystems. Would you rate our product and service?
 Here come GMP Grade Cytokines!Free Sample is available!
Here come GMP Grade Cytokines!Free Sample is available!  Here come GMP Grade Cytokines!Free Sample is available!
Here come GMP Grade Cytokines!Free Sample is available!
| Project Name | Project Stage | Molecule Type | Host Species | Therapeutic Area | Indications |
| CLDN18.2 CAR-T - 01 | PhaseI | Solid Tumor | Advanced refractory gastrointestinal tumors | ||
| CLDN18.2 VHH - 01 | Lead | Solid Tumor | Gastric Cancer,Pancreatic cancer |
| Project Name | Modality | Therapeutic Area | Indications | Stage | Right Available |
| Claudin18.2 mAb | Monospecific antibody | Oncology/Cancer | Gastric cancer | Phase I | Global |
| Claudin18.2×CD8+ T bsAb | Bispecific antibody | Oncology/Cancer | Gastric cancer,Pancreatic cancer,Oophoroma | IND | Global |
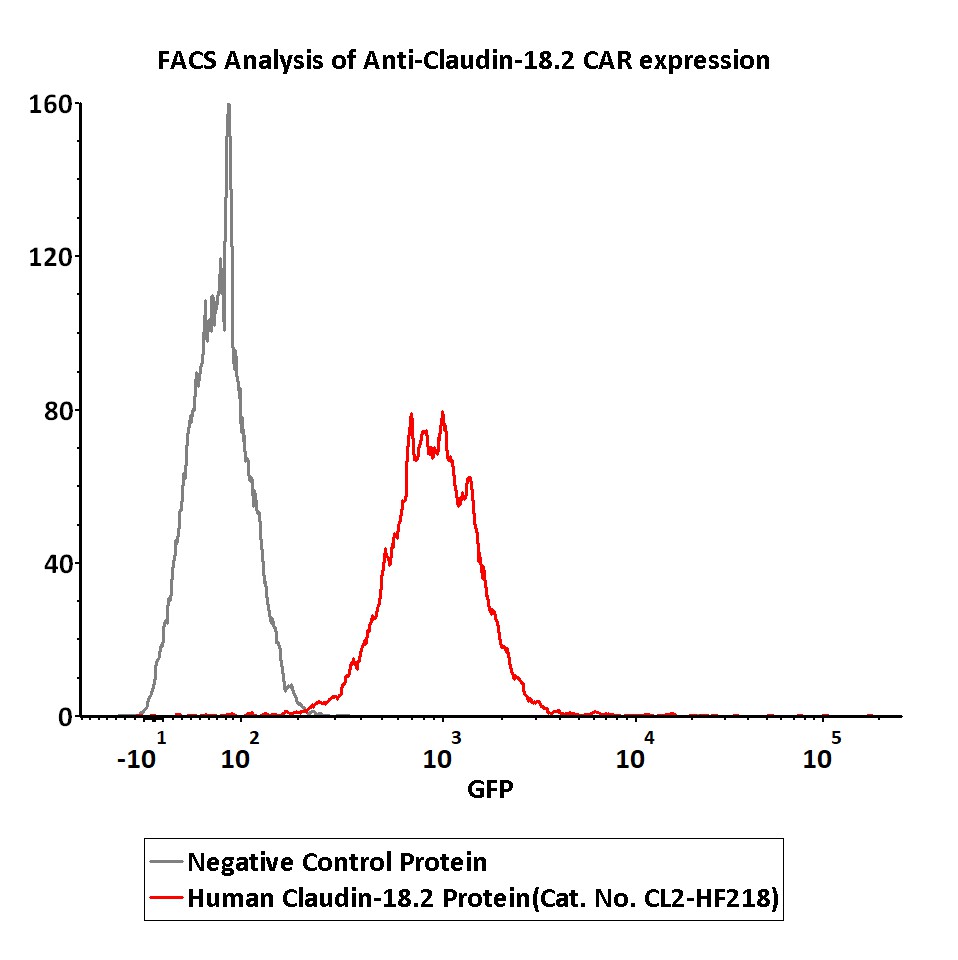
2e5 of Anti-Claudin-18.2 CAR-293 cells were stained with 100 μL of 3 μg/mL of Fluorescent Human Claudin-18.2 Full Length Protein-VLP (Cat. No.CL2-HF218) and negative control protein respectively, FITC signals was used to evaluate the binding activity (QC tested).

FACS assay shows that Anti-Claudin-18.2 antibody can bind to HEK293/Human Claudin-18.2 Stable Cell Line. HEK293/Human Claudin-18.2 stable cells was red line, Negative control HEK293 cells was grey line (QC tested).
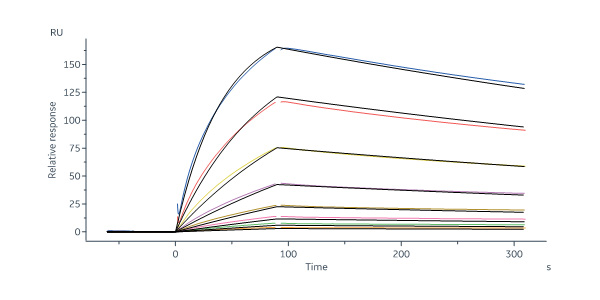
Monoclonal Anti-Chimeric Claudin-18.2 Antibody, Human IgG1 captured on Protein A Chip can bind Human / Cynomolgus Claudin-18.2, His,Twin-Strep Tag (Cat. No. CL2-H5586) with an affinity constant of 12.6 nM as determined in SPR assay (Biacore 8K) (Routinely tested).

Claudin-18.1 Monoclonal Antibody captured on CM5 chip via anti-mouse antibodies surface can bind Mouse Claudin-18.2, His,Twin-Strep Tag (Cat. No. CL2-M5585) with an affinity constant of 13 nM as determined in a SPR assay (in presence of DDM and CHS) (Biacore 8K) (Routinely tested).
| Name | Research Code | Research Phase | Company | Indications | Clinical Trials |
|---|---|---|---|---|---|
| BA-1105 | BA-1105 | Phase 1 Clinical | Shandong Boan Biotechnology Co Ltd | Solid tumours; Stomach Neoplasms; Esophageal Neoplasms; Pancreatic Neoplasms | Details |
| PM-3023 | PM-3023 | Phase 1 Clinical | Biotheus Inc | Solid tumours | Details |
| QL-1779 | QL-1779; QL1779 | Phase 1 Clinical | Qilu Pharmaceutical Co Ltd | Solid tumours | Details |
| JS-012 | JS-012 | Phase 1 Clinical | Shanghai Junshi Biosciences Co Ltd | Solid tumours | Details |
| RD-07 | RD-07 | Phase 1 Clinical | Peking University | Solid tumours | Details |
| Gresonitamab | AMG-910 | Phase 1 Clinical | Amgen Inc | Stomach Neoplasms | Details |
| Recombinant humanized anti-Claudin 18.2 monoclonal antibody (CARsgen) | AB-011 | Phase 1 Clinical | Kaixing Life Technology (Shanghai) Co Ltd | Solid tumours | Details |
| DR-30303 | DR30303 | Phase 1 Clinical | Zhejiang Doer Biologics Corp | Solid tumours | Details |
| KD-182 | KD-182 | Phase 2 Clinical | Nanjing Kaedi Biotechnology Co Ltd | Stomach Neoplasms; Carcinoma, Pancreatic Ductal | Details |
| BNT-141 | BNT-141 | Phase 2 Clinical | Biontech Se | Biliary Tract Neoplasms; Solid tumours; Esophageal Neoplasms; Stomach Neoplasms; Pancreatic Neoplasms; Cholangiocarcinoma; Esophageal adenocarcinoma; Neoplasm Metastasis; Adenocarcinoma | Details |
| M-108 | M108; M-108 | Phase 1 Clinical | FutureGen Biopharm (Beijing) Co Ltd | Solid tumours | Details |
| 124I-18B10 | 124-I-18-B-10; 124I-18B10 | Clinical | Transcenta Holding Ltd, Beijing Cancer Hospital | Gastrointestinal Neoplasms | Details |
| LY-011(Shanghai Longyao) | LY-011 | Phase 1 Clinical | Shanghai Longyao Biological Technology Co Ltd | Stomach Neoplasms; Pancreatic Neoplasms | Details |
| LB-1904 | LB-1904 | Phase 1 Clinical | Stomach Neoplasms | Details | |
| CT-048 | CT-048; KJ-C1807; KJ-C-1807 | Phase 1 Clinical | Esophageal Neoplasms; Stomach Neoplasms; Pancreatic Neoplasms; Adenocarcinoma | Details | |
| NBL-015 | NBL-015 | Phase 1 Clinical | Shanghai Xinshi Biological Medicine Co Ltd | Solid tumours; Stomach Neoplasms; Pancreatic Neoplasms; Neoplasms | Details |
| HEC-016 | HEC-016 | Phase 1 Clinical | People Hospital Of Luohu,Shenzhen | Esophageal Neoplasms; Stomach Neoplasms; Pancreatic Neoplasms | Details |
| IM-92 | IM-92 | Phase 1 Clinical | Beijing Immunochina Medical Science & Technology Co Ltd | Esophageal Neoplasms; Stomach Neoplasms; Pancreatic Neoplasms | Details |
| IBI-345 | IBI-345 | Phase 1 Clinical | Innovent Biologics(Suzhou) Co Ltd | Solid tumours | Details |
| SOT-102 | SOT102; SO-N102; SOT-102 | Phase 2 Clinical | Sotio Biotech AG | Stomach Neoplasms; Pancreatic Neoplasms; Digestive System Neoplasms | Details |
| Zolbetuximab | iMAB-362; GC-182 | Phase 3 Clinical | Ganymed | Lymphangioma, Cystic; Solid tumours; Pain; Stomach Neoplasms; Esophageal Neoplasms; Gastrointestinal Diseases; Pancreatic Neoplasms; Adenocarcinoma | Details |
| Q-1802 | Q-1802 | Phase 1 Clinical | Qiyu Biotechnology (Shanghai) Co Ltd | Solid tumours; Gastrointestinal Neoplasms | Details |
| Recombinant humanized monoclonal antibody MIL93(Mabworks) | MIL-93 | Phase 1 Clinical | Beijing Mabworks Biotech Co Ltd | Solid tumours; Neoplasms | Details |
| QLS-31905 | QLS-31905 | Phase 1 Clinical | Qilu Pharmaceutical Co Ltd | Solid tumours; Neoplasms | Details |
| IBI-360 | IBI-360 | Phase 1 Clinical | Innovent Biologics(Suzhou) Co Ltd | Solid tumours; Neoplasms | Details |
| IBI-389 | IBI-389 | Phase 1 Clinical | Innovent Biologics(Suzhou) Co Ltd | Solid tumours; Neoplasms | Details |
| JS-107 | JS-107 | Phase 1 Clinical | Shanghai Junshi Biosciences Co Ltd | Solid tumours; Neoplasms | Details |
| CMG-901(Connaught Biomedical Technology) | CMG-901 | Phase 1 Clinical | Keymed Biosciences Co Ltd | Solid tumours; Stomach Neoplasms; Esophageal Neoplasms; Pancreatic Neoplasms; Adenocarcinoma | Details |
| IMC-002(Suzhou Immunofoco) | IMC-002 | Phase 1 Clinical | Suzhou Immunofoco Biotechnology Co Ltd | Ovarian Neoplasms; Stomach Neoplasms; Esophageal Neoplasms; Pancreatic Neoplasms; Adenocarcinoma | Details |
| LM-302 | LM-302; TPX-4589 | Phase 2 Clinical | Eli Lilly Trading (Shanghai) Co Ltd | Solid tumours | Details |
| CLDN18.2 UCAR-T cell therapy | CLDN18.2 UCAR-T | Clinical | Zhongshan City People'S Hospital, Suzhou Maoxing Biotechnology Co Ltd | Stomach Neoplasms | Details |
| SKB-315 | SKB-315; SKB315 | Phase 1 Clinical | Sichuan Kelun-Biotech Biopharmaceutical Co Ltd | Solid tumours; Neoplasms | Details |
| KD-496 | KD-496(4th); KD-496 | Phase 1 Clinical | Nanjing Kaedi Biotechnology Co Ltd | Solid tumours; Stomach Neoplasms; Neoplasms; Pancreatic Neoplasms | Details |
| CT-041 | CT041 | Phase 2 Clinical | CARsgen Therapeutics Holdings Ltd | Solid tumours; Stomach Neoplasms; Esophageal Neoplasms; Pancreatic Neoplasms; Adenocarcinoma | Details |
| LM-102 | LM-102 | Phase 2 Clinical | LaNova Medicines Ltd | Solid tumours; Biliary Tract Neoplasms; Stomach Neoplasms; Esophageal Neoplasms; Neoplasms; Pancreatic Neoplasms | Details |
| SG-1906 | SG-1906; SG1906 | Phase 1 Clinical | Hangzhou Sumgen Biotechnology Co Ltd | Neoplasms | Details |
| BC-007 (Dragonboat Biopharmaceutical) | BC-007 | Phase 1 Clinical | Dragonboat Biopharmaceutical | Solid tumours; Neoplasms | Details |
| SHR-A-1904 | SHR-A-1904; SHR-A1904 | Phase 2 Clinical | Shanghai Hengrui Pharmaceutical Co Ltd | Solid tumours; Pancreatic Neoplasms; Neoplasms | Details |
| PM-1032 | PM1032; PM-1032 | Phase 1 Clinical | Biotheus Inc, Shanghai Genechem Co Ltd | Neoplasms | Details |
| Osemitamab | MSB-018; TST-001 | Phase 2 Clinical | Mabspace Biomedicine (Suzhou) Co Ltd, HJB (Hangzhou) Co Ltd | Solid tumours; Biliary Tract Neoplasms; Stomach Neoplasms; Esophageal Neoplasms; Neoplasms; Lung Neoplasms; Gallbladder Neoplasms | Details |
| LB-4330 | Bis-2; LB-4330 | Phase 1 Clinical | L&L Biopharma Co Ltd | Solid tumours | Details |
| IBI-343 | IBI-343 | Phase 1 Clinical | Innovent Biologics(Suzhou) Co Ltd | Solid tumours | Details |
| BC-008 | BC-008 | Phase 1 Clinical | Dragonboat Biopharmaceutical | Solid tumours; Neoplasms | Details |
| ASP-2138 | ASP-2138 | Phase 1 Clinical | Astellas Pharma Global Development Inc, Xencor Inc | Esophageal Neoplasms; Stomach Neoplasms; Pancreatic Neoplasms | Details |
| SPX-101 (Sparx Therapeutics) | SX-001; KY-71113; KY71113; SPX-101 | Phase 1 Clinical | Kpc Pharmaceuticals Inc | Solid tumours; Stomach Neoplasms | Details |
| RC-118 | RC-118; YH005 ADC | Phase 2 Clinical | RemeGen Co Ltd, Beijing Biocytogen Co Ltd | Solid tumours; Neoplasm Metastasis | Details |
| LCAR-C18S CAR-T cell therapy | LB-1908; LCAR-C18S; LCAR-C-18-S | Phase 1 Clinical | Nanjing Legend Biotechnology Co Ltd | Solid tumours; Stomach Neoplasms; Esophageal Neoplasms; Pancreatic Neoplasms | Details |
| ZL-1211 | ZL-1211 | Phase 2 Clinical | Zai Lab (Shanghai) Co Ltd | Solid tumours | Details |
| ASKB-589 | ASKB-589 | Phase 2 Clinical | Jiangsu Aosaikang Pharmaceutical Co Ltd | Solid tumours; Esophageal Neoplasms; Neoplasms; Pancreatic Neoplasms; Adenocarcinoma | Details |
| PT-886 | PT-886 | Phase 1 Clinical | Phanes Therapeutics Inc | Stomach Neoplasms; Pancreatic Neoplasms | Details |
| SYSA-1801 | SYSA-1801; SYSA1801; CPO-102; EO-3021 | Phase 1 Clinical | Jushi Biopharmaceutical Co Ltd | Solid tumours; Esophageal Neoplasms; Stomach Neoplasms; Pancreatic Neoplasms | Details |
| TJ-CD4B | TJ-CD4B; ABL-111; TJ-CLDN4B; TJ-CD4B/ABL111; TJ-033721 | Phase 1 Clinical | I-Mab Biopharma Co Ltd | Solid tumours; Stomach Neoplasms; Esophageal Neoplasms; Neoplasms; Esophageal adenocarcinoma; Carcinoma, Pancreatic Ductal; Neoplasm Metastasis | Details |
This web search service is supported by Google Inc.