 Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.  Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.
 Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free!  Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free!
 Thank you for choosing ACROBiosystems. Would you rate our product and service?
Thank you for choosing ACROBiosystems. Would you rate our product and service?  Thank you for choosing ACROBiosystems. Would you rate our product and service?
Thank you for choosing ACROBiosystems. Would you rate our product and service?
 Here come GMP Grade Cytokines!Free Sample is available!
Here come GMP Grade Cytokines!Free Sample is available!  Here come GMP Grade Cytokines!Free Sample is available!
Here come GMP Grade Cytokines!Free Sample is available!
> GMP Grade Cytokines
Consistent GMP products are crucial as key raw materials in cell therapy manufacturing. Adhering to regulatory guidelines, we strive to provide high-quality, safe, and reliable bioactive products for cell therapy manufacturing.
Develop your cell therapy manufacturing process with confidence by using GMP cytokines and growth factors. Our years of expertise in protein development and manufacturing, stringent quality control, and regulatory support allow us to offer industry-leading GMP proteins for ancillary use. We often use the same clone, sequence, and expression system as our research grade materials to make your switch to GMP as seamless as possible.


In response to the challenges impeding the growth of CGT industry, such as fluctuations in the quality of GMP critical materials, vulnerabilities in the supply chain, and regulatory uncertainties, ACROBiosystems has established the new GMP brand, Resilient Supply. This endeavor aims to provide global customers with more flexible, resilient, lower-risk, and higher-quality solutions, accompanied by enhanced professional support.
The essence of Resilient Supply is thoroughly embedded in the design and operational philosophy of ACROBiosystems' GMP facility. The facility features intelligent and modular design, boasting flexibility and resilience, capable of tackling complex and diverse production tasks to meet various supply demands for GMP-grade critical reagents.
Providing high-quality GMP products at an affordable cost can help lower the barrier in accessing innovative medicines including cell therapies. Our new facility was designed specifically for GMP production of proteins, enzymes, activation beads, and other solutions.
Want to learn more about
your GMP Protein options?
Quality control testing throughout the entire production process starting from bulk intermediates to the final lyophilized product ensures that our GMP grade products meets your needs and remains compliant. We ensure bioactivity, consistency, and stability in all our GMP solutions through our comprehensive quality management system.
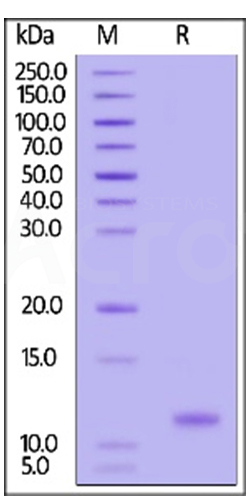
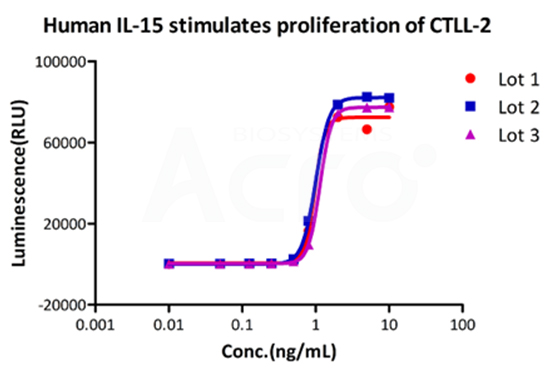

Three independent lots of GMP Human IL-15 (Cat. No. GMP-L15H13) were tested for the ability to simulate the proliferation of CTLL-2 cells. Average specific activity of GMP Human IL-15 was defined to be more than 0.8 x 107 IU/mg after calibration against human IL-15 WHO International Standard (NIBSC code: 95/554).
Human IL-2, GMP-grade
Human IL-7, GMP-grade
Human IL-15, GMP-grade
Explore >>
Request a protocol for GMP-grade IL-15 Bioactivity Verification
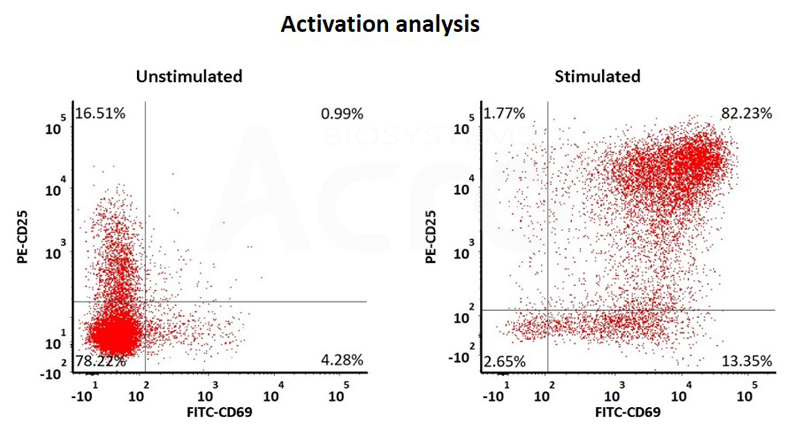
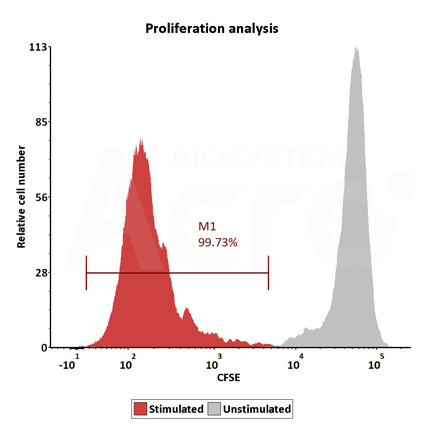
Human T cells were stimulated using GMP-grade ActiveMax Human T Cell Activation / Expansion CD3/CD28 beads (Cat. No. GMP-MBS001 ) for 24 hours. Activation was assessed by measuring expression of both activation markers CD25 and CD69 expression on T cell surface by staining with PE labeled anti-human CD25 antibody and FITC-labeled anti-human CD69 antibody respectively (QC tested).
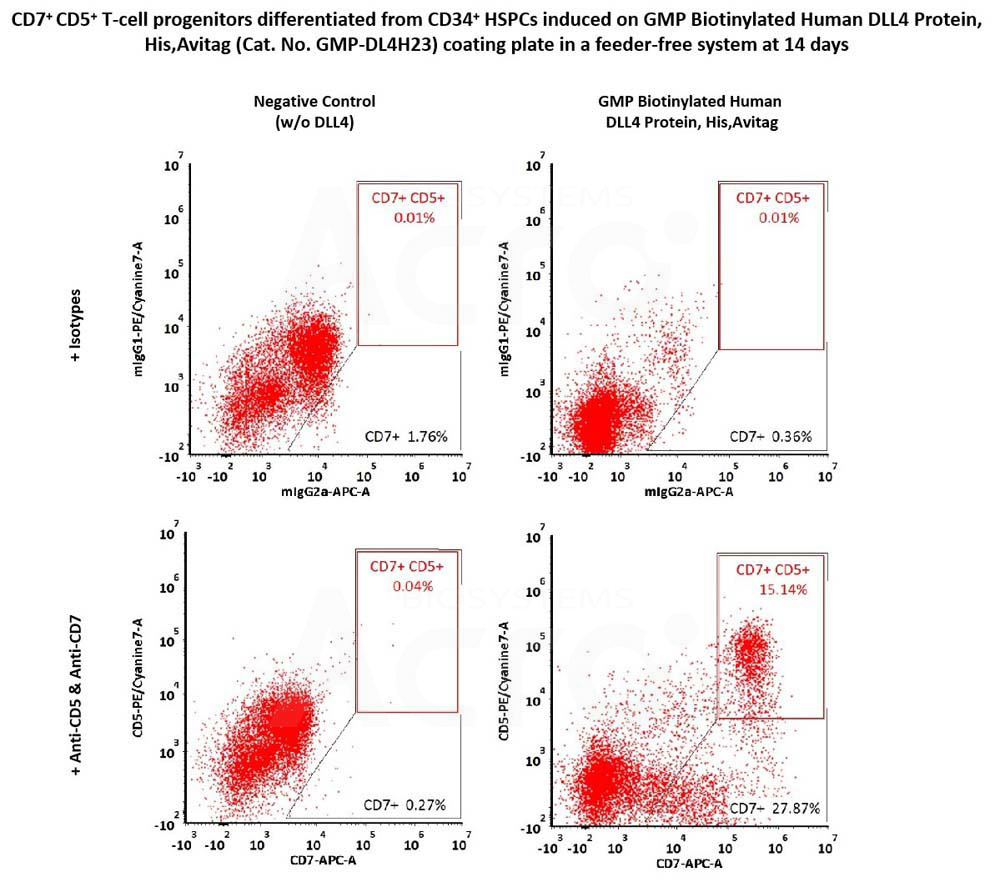
CD34+ CD45+ hematopoietic cells were seeded on GMP Biotinylated Human DLL4 Protein, His,Avitag (Cat. No. GMP-DL4H23) coated plates and differentiated for 14 days, then flow cytometry was used to detect the expression of T-cell progenitor markers, CD7 and CD5. The GMP Biotinylated Human DLL4 Protein, His,Avitag together with SCF, TPO, Flt3L and IL7, could induce the formation of CD7+ and CD7+ CD5+ T-cell progenitors (Routinely tested).
DLL4, Fc tag, GMP-grade
Biotinylated DLL4, His, Avitag, GMP-grade
VCAM-1, GMP-grade
Explore >>

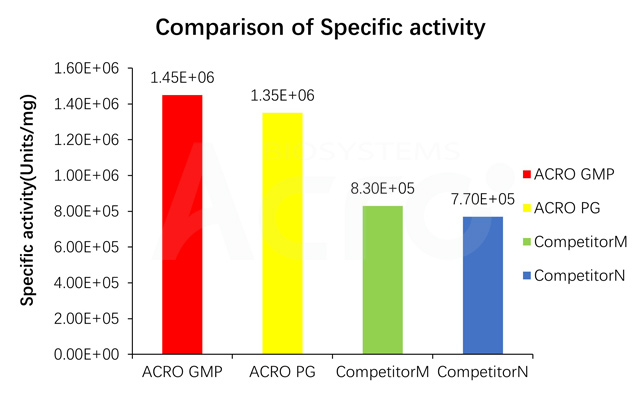
Specific activity for GMP GENIUS Nuclease is measured under standard assay conditions. The specific activity of GMP GENIUS Nuclease, is >1.2E+06 unit/mg protein. One unit will digest sonicated salmon sperm DNA to acid-soluble oligonucleotides equivalent to a ΔA260 of 1.0 in 30 min at pH 8.0 at 37 °C, which corresponds approximately to complete digestion of 37 μg DNA.
Salt-Active GENIUS Nuclease, GMP-grade
NLS-Cas9 Nuclease, GMP-grade
Explore >>
Interested in testing
a GMP grade product ?
Search for the solutions that fit your needs by your application! We currently offer a wide array of products designed to help with cell manufacturing of immune cells including T cells, NK cells, and Dendritic cells. We also offer an increasingly large catalog of iPSC growth factors and differentiation factors. This includes recombinant proteins such as DLL4, VCAM-1, and many others. Basement membrane extracts and extracellular matrix proteins are also available.
Know exactly what you’re looking for? Explore our catalog of solutions to find what’s right for you. Our GMP-grade solutions are manufactured under regulatory guidelines for ancillary materials in cell therapy manufacturing processes. Related Premium (Pre-GMP) grade solutions are also listed to help you find related products to help you streamline your transition to GMP.
Learn More About our Transitioning to GMP grade Solutions 
Learn More About our GMP Capabilities, Quality, and Regulatory Support 
| Molecule | Source | GMP Grade Catalog No. | DMF Filed for GMP Grade | Premium (Pre-GMP) Grade Catalog No. |
|---|
*Already purchased a DMF-filed protein? Click here to apply for your DMF authorization.
Can’t find the
GMP Product you need?

In this 15-minute webinar, learn about how selecting the right raw material supplier to partner with is central to ensuring end-users' primary considerations of patient safety, lot-to-lot consistency, and supply chain reliability.
We are committed to developing high quality, key reagents for use in clinical studies and commercialization of cell and gene therapies. Following current pharmaceutical manufacturing practices along with more stringent quality management and release testing standards, we strive to provide our customers with the best GMP grade products. We are continuously improving our GMP quality management system to make better raw materials that meet the global regulatory requirements for cell and gene therapy manufacturing. We welcome all customer audits and regularly audit our own facilities to ensure compliance.
Explore the contents of our regulatory support files (RSF) and apply here!
Each GMP protein is manufactured and tested in a GMP-compliant facility. Our GMP quality management system was developed and maintained in accordance with relevant cGMP and GMP guidelines.
Key highlights include:
Each GMP protein is manufactured and tested in a GMP-compliant facility. Our GMP quality management system was developed and maintained in accordance with relevant cGMP and GMP guidelines.
Want to learn more about
your GMP Protein options?
Adventitious agents contaminants within the final product of cell and gene therapies are a significant point of concern. To minimize external contaminants, a strict quality control system is necessary. GMP grade products refer to several GMP guidelines outlined by regulatory agencies and are performed periodically throughout the production and quality control process.
This includes:
Our professional regulatory support team has experience working with therapeutic manufacturers across the globe, with customers in China, United States, South Korea, and more. CDE, FDA, and KFDA declarations have been submitted with our support. We provide the Regulatory support file documentation to assist customers at different phases of development and use for declaration requirements.
Learn More About our GMP Facilities & Capabilities 
Want to learn more about
your GMP Protein options?
When it comes to preparing the transition from RUO to GMP grade proteins, it can be hard to know exactly when to start switching. We answer a few of the more common questions that we get in the industry to help shed some insight about the regulatory landscape of utilizing GMP raw materials and enter the clinical phase without any worries.
Learn more about making the transition to GMP here!
Raw materials should be sourced from qualified suppliers following a pre-established supplier qualification and monitoring process. We perform annual risk assessments for raw materials used in our GMP process along with our suppliers. This encompasses visual inspections and documentation tracking of associated documents including certificates of analysis (CoA), Certificates of Origin (CoO), TSE/BSE declarations, and animal-free statements, when applicable. Before acceptance, key raw materials are always identified and validated.
We advise cell therapy manufacturers to source either pharmaceutical or GMP-grade whenever possible. Our GMP-grade products are manufactured and tested in accordance with relevant international standards. * Raw material suppliers should have a certified quality management system (QMS) along with an ISO 9001 / 13485 certification. Independent audits, quality policies, and quality processes should be established to ensure quality that matches customer expectations. Cell therapy manufacturers should also perform due diligence by qualifying their suppliers through questionnaires, on-time delivery, SCAR, and other auditing activities. Documentation including certificates of analysis, certificates of origin, and other audit trail documentation should be received from each supplier and kept as records.
Cell therapy manufacturers should begin discussions with potential suppliers as soon as possible to avoid issues when entering production. Ensuring raw material suppliers have the ability to scale-up can prevent any last-minute changes that could require expensive revalidation studies.
Identifying a secondary supplier is also crucial. Although raw materials may look identical, there is no guarantee on how it behaves in your biological system. Do not assume that switching suppliers can be easily done – subsequent validation studies showing equivalence between raw materials from different suppliers is required. Identifying a secondary supplier can be an insurance policy if any issues arise with your primary supplier.
*International standards include:
The quality of manufactured cell and gene therapies is directly influenced by the raw materials used in their production, which, in turn, impacts the therapy’s impact and efficacy. Relevant regulations stipulate that the selection of raw materials should consider their necessity, rationality, and safety, preferably opting for materials approved for human use or meeting pharmacopoeia standards. Although safety and quality requirements for raw materials have lower priority in the initial preclinical stage, transitioning to regulatory-compliant GMP-grade materials becomes imperative during CMC or clinical conversion. This transition involves thorough testing, safety assessments, and process validation, demanding considerable time and effort.
If the same supplier is utilized, the physical properties and biological activity of premium-grade (PG) raw material ideally mirror those of GMP-grade, differing mainly in regulatory support documentation. Rigorous safety testing of materials interacting with patients is crucial, and confirming the consistency of manufacturing processes between PG and GMP materials is recommended. We ensure that both our PG and GMP products are derived from the same cell bank source, undergo strict quality control, demonstrating biological identity through extensive trials.
For clinical applications, an early shift to GMP is recommended, even during the preclinical phase, facilitating equivalence testing at a more manageable stage. Opting for an experienced supplier well-versed in quality management, protein biochemistry, analytical testing, and regulatory affairs is advantageous. Experienced suppliers aid in navigating changing regulatory environments, providing necessary documentation like a Drug Master File (DMF) to streamline regulatory processes. Audits of supplier facilities, preferably in person or online, strengthen the strategic partnership between suppliers and customers. Maintaining strict adherence to approved operating procedures, at least for three consecutive batches, ensures consistency and compliance with predetermined acceptance criteria for our PG products developed through the GMP product line.
Biological reagents are notorious when it comes to susceptibility to variability, especially cytokines and growth factors. Only after these reagents have been successfully developed and validated through multiple successive batches meeting the stringent outlined specifications, along with the establishment of a stable process and rigorous conformance assessment, can the product be moved into large-scale manufacturing and commercialized. This process forms a robust foundation in ensuring the consistency and reliability of GMP raw materials.
Furthermore, the manufacturing of products occurs within GMP facilities and under GMP quality systems, with strict control implemented through documented Standard Operating Procedures (SOPs) and trained personnel. This crucial information should be disclosed during audits. It is reasonable to request data from multiple batches to evaluate a supplier's capability to consistently produce a reproducible protein. Ideally, materials should be sampled from various batches to assess their consistency across the entire system. We identify standard batches and compare each new production batch to the established standard before releasing it to the market. This approach mitigates variability and ensures consistent product performance. Currently, we maintain excellent control over lot-to-lot differences, specifically in biological activity, within the range of 10-15%.
Safety regarding any raw materials employed in cell and gene therapy manufacturing is critical to ensure final therapeutic quality. This emphasizes the need for safety control to prevent contamination by exogenous factors. These ‘exogenous’ factors refer to inoculants, cell matrices, residual raw materials during production, bacteria, fungi, mycoplasma, and viruses.
The FDA recommendation of using the highest-quality materials means that all raw material suppliers should ensure that their materials or reagents are produced under the appropriate quality standards to mitigate any unreasonable risks to patients. This means that strict quality control of raw materials used in CGT production is a necessary measure to reduce the risk of external contamination and ensure the safety and efficacy of cell and gene therapy products. Expanding upon this requirement, the following requirements are outlined in various international pharmacopeias, including:
Making the Transition to GMP Brochure 
The Balancing Act: Why Finding the Right Time to Introduce GMP Is Your Secret to Success 
Quality Management and Safety Evaluations for GMP 
Understanding Global GMP Regulatory Guidelines 
GMP Regulations Frequently Asked Questions 
Infographic - Production Process & Quality Control Workflow 
Qualifying Raw Materials for Cell Therapy Manufacturing 
Special Topic on Deep Interpretation of GMP Product Quality 1 
Looking for your next
GMP Supplier?
This web search service is supported by Google Inc.