 Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.  Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.
 Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free!  Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free!
 Thank you for choosing ACROBiosystems. Would you rate our product and service?
Thank you for choosing ACROBiosystems. Would you rate our product and service?  Thank you for choosing ACROBiosystems. Would you rate our product and service?
Thank you for choosing ACROBiosystems. Would you rate our product and service?
 Here come GMP Grade Cytokines!Free Sample is available!
Here come GMP Grade Cytokines!Free Sample is available!  Here come GMP Grade Cytokines!Free Sample is available!
Here come GMP Grade Cytokines!Free Sample is available!
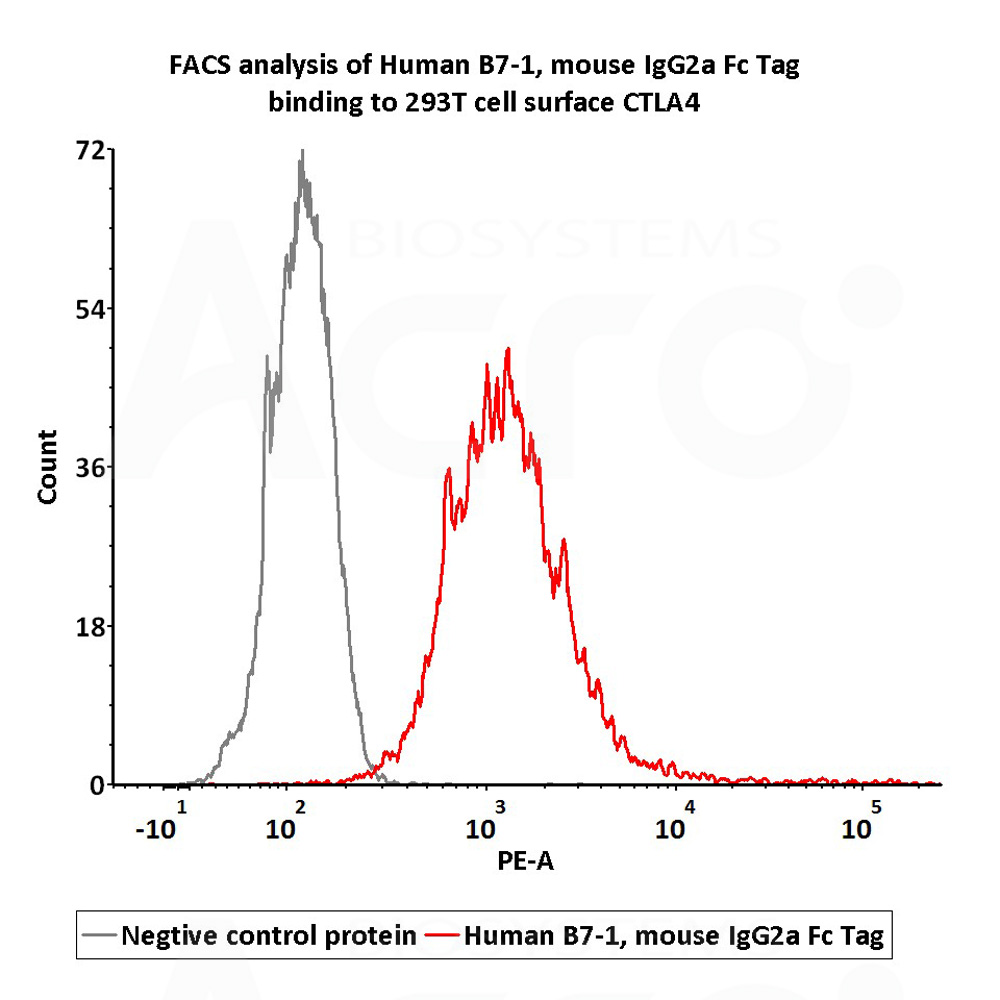
Flow Cytometry assay shows that Human B7-1, Mouse IgG2a Fc Tag (Cat. No. B71-H52A4) can bind to 293 cell overexpressing human CTLA-4. The concentration of Human B7-1 is 1 μg/mL (Routinely tested).

FACS analysis shows that the binding of Human B7-1, Mouse IgG2a Fc Tag (Cat. No. B71-H52A4) to 293 overexpressing CTLA-4 was inhibited by increasing concentration of neutralizing Anti-human CTLA-4 antibody. The concentration of Human B7-1 is 1 μg /mL. The IC50 is 5.5 μg/mL (Routinely tested).
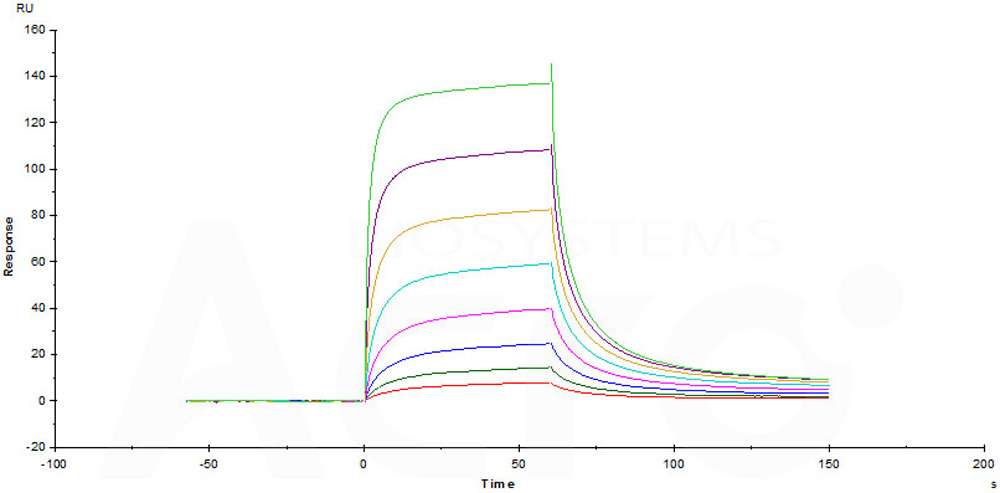
Immobilized Human / Cynomolgus / Rhesus macaque CD28, Fc Tag (Cat. No. CD8-H525a) on CM5 Chip can bind Human B7-1, Mouse IgG2a Fc Tag (Cat. No. B71-H52A4) with an affinity constant of 2.72 μM as determined in a SPR assay (Biacore T200) (Routinely tested).
| Name | Research Code | Research Phase | Company | First Brand Name | First Approved Country | First Indication | First Approved Company | First Approved Date | Indications | Clinical Trials |
|---|---|---|---|---|---|---|---|---|---|---|
| Belatacept | LEA29Y; LEA-029; BMS-224818; L104EA29YIg | Approved | Bristol-Myers Squibb Company | Nulojix | EU | Rejection of renal transplantation | Bristol-Myers Squibb Pharma Eeig | 2011-06-15 | Rejection of renal transplantation; Diabetes Mellitus, Type 1; Arthritis, Rheumatoid; Pancreatic neuroendocrine tumors (pNET); Proteinuria; Rejection of organ transplantation; Delayed Graft Function; Rejection in heart transplantation; Kidney Failure, Chronic | Details |
| Abatacept | ONO-4164; BMS-188667; BMS-188667SC; ONO-4164SC | Approved | Bristol-Myers Squibb Company | Orencia | United States | Graft vs Host Disease | Bristol Myers Squibb Srlcompany | 2005-12-23 | Arthritis, Psoriatic; Arthritis; Arthralgia; Common Variable Immunodeficiency; Cardiovascular Diseases; Sjogren-Larsson Syndrome; Urticaria; Asthma; Colitis, Ulcerative; Nephrotic Syndrome; Lupus Erythematosus, Systemic; Takayasu Arteritis; Psoriasis; Anemia, Aplastic; Glomerulosclerosis, Focal Segmental; Mucopolysaccharidosis I; Polymyalgia Rheumatica; Multiple Sclerosis; Leukocyte-Adhesion Deficiency Syndrome; Uveitis; Granulomatous Disease, Chronic; Lymphohistiocytosis, Hemophagocytic; Scleroderma, Diffuse; Inflammation; Myocarditis; Eye Diseases; Muscular Diseases; Crohn Disease; Behcet Syndrome; Kostmann Syndrome; Anemia, Sickle Cell; Thrombasthenia; Vitiligo; Spondylitis, Ankylosing; Anti-Neutrophil Cytoplasmic Antibody-Associated Vasculitis; Myasthenia Gravis; Granulomatosis with Polyangiitis; Wiskott-Aldrich Syndrome; Polymyositis; Diabetes Mellitus, Type 1; Multiple Sclerosis, Relapsing-Remitting; Hematologic Diseases; Alopecia Areata; Giant Cell Arteritis; Myositis; Dermatomyositis; Immunoglobulin G | Details |
| Name | Research Code | Research Phase | Company | Indications | Clinical Trials |
|---|---|---|---|---|---|
| Membrane bound ligand T-SIGn virus | NG-348 | Phase 1 Clinical | Psioxus Therapeutics Ltd | Neoplasms | Details |
| rVV-740CTA vaccine (University Hospital Basel) | rVV-740CTA | University Hospital Basel | Details | ||
| CTLA-4/FasL fusion protein (KAHR Medical) | KAHR-102 | Kahr Medical | Details | ||
| Belatacept biosimilar (Alphamab) | KN-019 | Phase 2 Clinical | Rejection of renal transplantation; Arthritis, Rheumatoid | Details | |
| Chimeric antigen receptor T-cell therapy (Hunan Zhaotai Yongren Biotech) | Z-CTLs | Phase 1 Clinical | Hunan Zhaotai Yongren Biotech Co Ltd | Carcinoma, Non-Small-Cell Lung | Details |
| Recombinant human CTLA-4-FC fusion protein(Beijing Vdjbio) | Phase 1 Clinical | Beijing Vdjbio Co Ltd | Arthritis, Rheumatoid | Details | |
| ORCA-010 | ORCA-010 | Phase 2 Clinical | Orca Therapeutics Bv | Prostatic Neoplasms | Details |
This web search service is supported by Google Inc.